Leucine-rich alpha-2-glycoprotein 1 (LRG1) as a novel ADC target†
Abstract
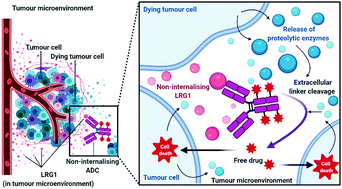
Leucine-rich alpha-2-glycoprotein 1 (LRG1) is present abundantly in the microenvironment of many tumours where it contributes to vascular dysfunction, which impedes the delivery of therapeutics. In this work we demonstrate that LRG1 is predominantly a non-internalising protein. We report the development of a novel antibody–drug conjugate (ADC) comprising the anti-LRG1 hinge-stabilised IgG4 monoclonal antibody Magacizumab coupled to the anti-mitotic payload monomethyl auristatin E (MMAE) via a cleavable dipeptide linker using the site-selective disulfide rebridging dibromopyridazinedione (diBrPD) scaffold. It is demonstrated that this ADC retains binding post-modification, is stable in serum and effective in in vitro cell studies. We show that the extracellular LRG1-targeting ADC provides an increase in survival in vivo when compared against antibody alone and similar anti-tumour activity when compared against standard chemotherapy, but without undesired side-effects. LRG1 targeting through this ADC presents a novel and effective proof-of-concept en route to improving the efficacy of cancer therapeutics.
- This article is part of the themed collection: RSC Chemical Biology Editors' Choice


 Please wait while we load your content...
Please wait while we load your content...