Exosomes from human cord blood plasma accelerate cutaneous wound healing by promoting fibroblast function, angiogenesis, and M2 macrophage differentiation
Abstract
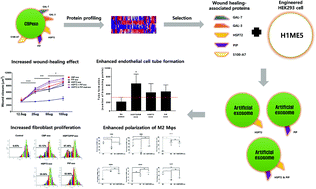
Exosomes contain natural cargo molecules, such as miRNA, mRNA, and proteins, and transfer these functional cargos to neighboring or distant cells through circulation. In the wound-healing process, exosomes in the human blood and body fluids perform various functions, including proliferation, angiogenesis, differentiation, and wound healing, owing to their unique compositions. However, there is very limited information on the wound-healing effect of proteins in human cord blood plasma exosomes (CBPexo). Therefore, we studied the wound-healing potential of these proteins in terms of fibroblast functions, angiogenesis, and M2 macrophage differentiation. When scratch wound assays were conducted using human fibroblasts, CBPexo exhibited better wound-healing effects than adult blood plasma exosomes (ABPexo). CBPexo also promoted angiogenesis and differentiation of M2 macrophages, thus promoting the transition from inflammation to proliferation. To evaluate the CBPexo molecules involved, five proteins, GAL-3, GAL-7, HSP-72, PIP, and S100-A7, were selected through proteomic analysis, and their functions were investigated using an artificial exosome that expresses these proteins. Among these, HSP72 and PIP exhibited wound-healing effects similar to CBPexo. Furthermore, artificial exosomes expressing both HSP72 and PIP showed better wound-healing effects than CBPexo. Therefore, the use of artificial CBPexo can potentially overcome the limitations related to exosome production from CB.


 Please wait while we load your content...
Please wait while we load your content...