The impact of cation and anion pairing in ionic salts on surface defect passivation in cesium lead bromide nanocrystals†
Abstract
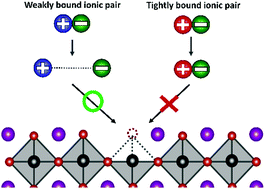
Imperfect passivation of surface charge traps on metal halide perovskite (MHP) nanocrystals remains a key obstacle to achieving higher performance in optoelectronic devices. Due to the strong ionic nature of MHPs, ionic salts have been identified as effective surface charge trap passivating ligands. In this study, based on photoluminescence quantum yield (PLQY) and time-resolved photoluminescence (TRPL) measurements on cesium lead bromide nanocrystals (CsPbBr3 NCs), we find that the pairing between cation and anion of an ionic salt results in a significant impact on trap passivation. Using density of functional theory (DFT) calculations, we identify the binding interaction between the cation and anion of the ionic pair to be a major factor in determining the trap passviation efficacy.


 Please wait while we load your content...
Please wait while we load your content...