Quinoidal thioalkyl-substituted bithiophene small molecule semiconductors for n-type organic field effect transistors†
Abstract
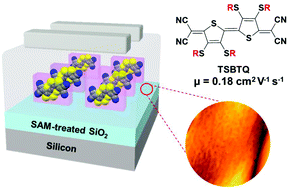
Two tetrathioalkyl-substituted bithiophene-based small molecule quinoids (TSBTQs) having different chain lengths (thio-hexyl and -decyl) are synthesized and applied as an n-type active component in organic field effect transistors (OFETs). The resulting two TSBTQs exhibit good solubility in various organic solvents and LUMO levels below −4.0 eV. The theoretical DFT calculation supported by single crystal structures confirms the π-conjugated backbone planarity due to the S(thiophene)⋯S(alkyl) non-covalent interaction. Optimized TSBQT-10 OFETs exhibit an electron mobility of 0.18 cm2 V−1 s−1, which is higher than that of TSBTQ-6 (0.09 cm2 V−1 s−1). The reliability of the OFETs under representative environmental and operational conditions is also determined. The effects of side chains including their lengths and contribution to the main chain π-system coplanarity presented here demonstrate an efficient method to manipulate the charge carrier mobility of the quinoidal organic semiconductors.
- This article is part of the themed collection: Celebrating Tobin Marks’ 75th Birthday


 Please wait while we load your content...
Please wait while we load your content...