General strategy for tuning the Stokes shifts of near infrared cyanine dyes†
Abstract
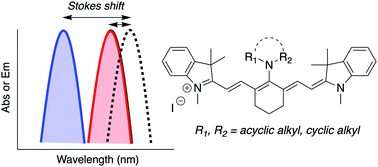
We report a significant Stokes shift enhancement in near-infrared fluorescing cyanines as a result of C4′-substitution with cyclic or acyclic amines. Based on a combined experimental and density functional study, a simple strategy for optimizing the Stokes shift is proposed. By tuning the relative energies of cyanine-like and bis-dipolar conformers, differing in the rotational angle of the amine substituent, it is possible to develop molecules that undergo conformational change upon excitation, resulting in a predictable Stokes shift.


 Please wait while we load your content...
Please wait while we load your content...