Y-shaped tricatenar azobenzenes – functional liquid crystals with synclinic–anticlinic transitions and spontaneous helix formation†
Abstract
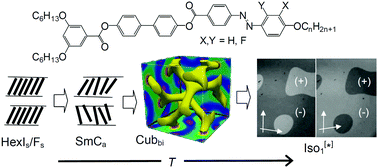
A series of achiral tricatenar rod-like molecules with a 3,5-disubstitution pattern at one end and a single alkyl chain at the other end of a rod-like azobenzene derived core is reported. Depending on temperature and alkyl chain length, these Y-shaped compounds self-assemble into different types of liquid crystalline (LC) phases, ranging from non-tilted and synclinic tilted hexatic, via non-tilted and anticlinic tilted smectic and bicontinuous cubic LC phases, to a spontaneous mirror symmetry broken isotropic liquid (Iso1[*]) or a related achiral liquid network phase (Iso1). An additional tilted, but uniaxial smectic phase was observed at the transition between anticlinic and synclinic tilt correlation and was investigated by soft resonant X-ray scattering with respect to possible helix formation. This work provides a new concept for the design of technological interesting azobenzene based LC materials with anticlinic tilted smectic C phases (SmCa) and with azobenzene units organized in the long range or short range helical network structures of bicontinuous cubic and chiral isotropic liquid phases, respectively. Core fluorination removes all lamellar phases, leaving only the cubic phase over wide temperature ranges, even at ambient temperature.


 Please wait while we load your content...
Please wait while we load your content...