Methoxylation of quinoidal bithiophene as a single regioisomer building block for narrow-bandgap conjugated polymers and high-performance organic field-effect transistors†
Abstract
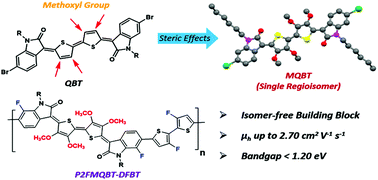
Quinoidal bithiophene (QBT) is a highly attractive building block for high-performance polymeric semiconductors. However, it is a challenge to prepare a single regioisomer of a QBT-based building block using an indophenine reaction. Here, we introduce steric hindrance of the methoxyl group in a QBT-based system, which ensures the stereospecificity of the resulting monomers. Both methoxylated QBT (MQBT) and fluorinated MQBT (2FMQBT) monomers are copolymerized with 2,2′-bithiophene and 3,3′-difluoro-(2,2′-bithiophene) (DFBT) to form four donor–acceptor (D–A) copolymers, which display a narrow optical bandgap (<1.20 eV). Because of the introduction of fluorine atoms, P2FMQBT-DFBT displays good coplanarity and close solid-state packing, possessing the highest hole mobility of up to 2.70 cm2 V−1 s−1, which represents one of the best values for quinoidal polymers. Most importantly, the preparation of single regioisomers of MQBT and 2FMQBT is synthetically feasible for creating new QBT-based high-performance polymers.
- This article is part of the themed collections: Celebrating Tobin Marks’ 75th Birthday and Journal of Materials Chemistry C Lunar New Year collection 2021


 Please wait while we load your content...
Please wait while we load your content...