Effects of alkoxylation position on fused-ring electron acceptors†
Abstract
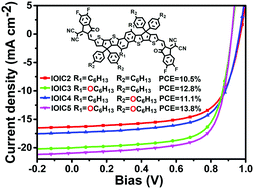
Four fused-ring electron acceptors composed of the same naphtho[1,2-b:5,6-b′]dithiophene-based core and 3-(1,1-dicyanomethylene)-5,6-difluoro-1-indanone end groups without or with hexyloxyl groups on the core and/or phenyl side chains are compared to systematically study the effects of alkoxylation position on the molecular packing, optical, electronic, and photovoltaic properties of the nonfullerene acceptors. Alkoxylation on the core red-shifts absorption and reduces bandgap, while that on side chains has little effect on absorption and bandgap. Alkoxylation on the core up-shifts the HOMO and down-shifts the LUMO, while that on side chains shows very little effect on the energy levels. Alkoxylation on the core slightly improves electron mobility relative to that on the side chains. Both methods of alkoxylation decrease open-circuit voltage, but increase short-circuit current density and fill factor, leading to improved efficiencies of the organic solar cells. Finally, when blended with the polymer donor PM6, IOIC3/IOIC4 with alkoxylation on the core or side chains yields efficiencies of 11.1–12.8%, which are higher than that of IOIC2 without alkoxylation (10.5%). IOIC5 with alkoxylation on both the core and side chains yields the highest efficiency of 13.8%.
- This article is part of the themed collections: Celebrating Tobin Marks’ 75th Birthday and Journal of Materials Chemistry C Lunar New Year collection 2021


 Please wait while we load your content...
Please wait while we load your content...