Specific features of the electronic and crystal structure of CuxZrSe2 (0 < x ≤ 0.3)†
Abstract
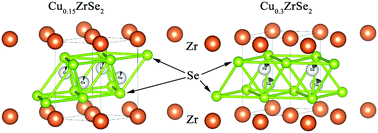
Crystal and electronic structure, optical absorption and transmission spectra, temperature dependences of conductivity and magnetic susceptibility were studied for copper intercalated ZrSe2 in the copper concentration range of 0 ≤ x ≤ 0.3. The electronic structure of CuxZrSe2 single crystals was studied at room temperature using X-ray Absorption Near Edge Structure spectroscopy (XANES), resonant photoelectron spectroscopy (ResPES) and optical absorption. The partial and total densities of states were calculated to determine the atomic contributions to the valence band. The competition between the occupation of octahedral and tetrahedral sites in the van der Waals gap by copper was found. The contribution of both positions to the formation of the chemical bond between copper and the ZrSe2 lattice with the domination of the Cu–Se interaction was determined. It was found that the intercalation of copper in the concentration range of x = 0–0.2 resulted in the formation of the localized impurity states in the Se 4p/Zr 4d band gap but not in the charge transfer to the conduction band. As the copper concentration increased above x = 0.2, the semiconductor-to-metal transition occurred due to the reaching of the conduction band by the Fermi level. The Se 4p/Zr 4d band gap was found to be a direct one in the whole studied concentration range. Some inconsistencies in the conductivity and spectral data along with the DOS calculation results were explained by the Frenkel defects in the ZrSe2 lattice.


 Please wait while we load your content...
Please wait while we load your content...