High-mobility organic single-crystalline transistors with anisotropic transport based on high symmetrical “H”-shaped heteroarene derivatives†
Abstract
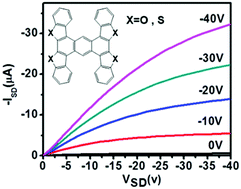
To reveal the influence of synergistic effect of non-covalent forces on the FET performance, two highly symmetrical “H”-shaped heteroarene derivatives anthra[2,1-b:3,4-b′:6,5-b′′:7,8-b′′′]tetra(benzothiophene) (ATBT) and anthra[2,1-b:3,4-b′:6,5-b′′:7,8-b′′′]tetra(benzofuran) (ATBF) as novel two-dimensional (2D) organic semiconductor materials were synthesized. The thermal, optical and electrochemical properties of ATBT and ATBF were investigated and high stability was confirmed. Single crystal XRD of ATBT confirmed the close π–π stacking due to the “wider” π-conjugation framework and four S atoms sited on both sides of each molecule could produce eight S⋯S contacts with neighbouring molecules. Mobility of up to 15.6 cm2 V−1 s−1 could be achieved for the single-crystalline field effect transistor, which was fabricated by the ‘two-dimensional organic-ribbon mask’ technique based on the individual ATBT microribbon. The strong anisotropy along different crystal axes is consistent with the molecular arrangement, which was evidenced by XRD, TEM and corresponding selected-area electron diffraction pattern. Moreover, the analogue ATBF shows lower FET performance due to lack of S⋯S contacts in comparison to that of ATBT, which reflects that the synergistic effect of non-covalent forces has an important influence on the molecular aggregation and electrical properties.


 Please wait while we load your content...
Please wait while we load your content...