Photostable orange-red fluorescent unsymmetrical diketopyrrolopyrrole–BF2 hybrids†
Abstract
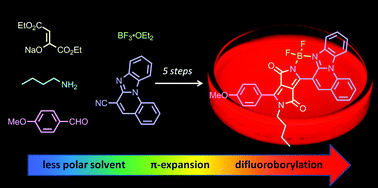
The straightforward synthesis of structurally unique DPP–BODIPY hybrids has been developed using unsymmetrical, imidazopyridine substituted DPPs. These hybrids exhibit a superb combination of photophysical properties including high photostability, good fluorescence quantum yield as well as markedly bathochromically shifted absorption and emission compared to conventional diketopyrrolopyrroles. Increasing the size of the imidazopyridine substituent and/or the electron donating power of the other aryl substituent can further redshift both absorption and emission to reach ∼650 nm for the free-base and ca. 700 nm for boron-chelates. A strong intramolecular hydrogen bond is responsible for the small change in geometry between the ground and excited states and hence relatively small differences in photophysical properties upon formation of boron-chelates are observed. The solvent dependence of the photophysical properties for the free base and DPP–BF2 complexes were investigated and show strong fluorescence with long lifetimes in both non-polar and polar aprotic environments.


 Please wait while we load your content...
Please wait while we load your content...