Nitrile-substituted 2-(oxazolinyl)-phenols: minimalistic excited-state intramolecular proton transfer (ESIPT)-based fluorophores†
Abstract
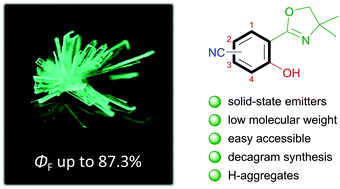
Herein, we present minimalistic single-benzene, excited-state intramolecular proton transfer (ESIPT)-based fluorophores as powerful solid-state emitters. The very simple synthesis gave access to all four regioisomers of nitrile-substituted 2-(oxazolinyl)-phenols (MW = 216.1). In respect of their emission properties they can be divided into aggregation-induced emission enhancement (AIEE) luminophores (1-CN and 2-CN), dual state emission (DSE) emitters (3-CN) and aggregation-caused quenching (ACQ) fluorophores (4-CN). Remarkably, with compound 1-CN we discovered a minimalistic ESIPT-based fluorophore with extremely high quantum yield in the solid state ΦF = 87.3% at λem = 491 nm. Furthermore, quantum yields in solution were determined up to ΦF = 63.0%, combined with Stokes shifts up till 11.310 cm−1. Temperature dependent emission mapping, crystal structure analysis and time-dependent density functional theory (TDDFT) calculations gave deep insight into the origin of the emission properties.
- This article is part of the themed collection: 2020 Journal of Materials Chemistry C most popular articles


 Please wait while we load your content...
Please wait while we load your content...