tert-Butyl substituted hetero-donor TADF compounds for efficient solution-processed non-doped blue OLEDs†
Abstract
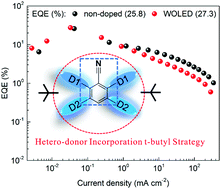
For the development of solution-processed organic light-emitting diodes (OLEDs), it is highly desirable yet challenging to realize solution-processable non-doped thermally activated delayed fluorescence (TADF) emitters due to their high efficiency and excellent compatibility to the wet methods. Herein, two pairs of blue TADF isomers are designed and synthesized with a hetero-donor configuration for the realization of high photoluminescent quantum yield. The incorporation of two tert-butyl groups in the molecules can effectively increase the molecular solubility and reduce the aggregation-caused self-quenching of excitons in neat films by inhibiting the intramolecular vibrational relaxation and the intermolecular π–π stacking. Solution-processed non-doped OLEDs are achieved with these blue TADF emitters, exhibiting the record-high external quantum efficiencies (EQE) of 25.8%. Furthermore, an all-TADF white OLED with an EQE of 27.3% is also achieved by employing a single emitting layer with the blue TADF emitter as a host for an orange-red TADF dopant.


 Please wait while we load your content...
Please wait while we load your content...