Effects of alkyl chain length and anion on the optical and electrochemical properties of AIE-active α-cyanostilbene-containing triphenylamine derivatives†
Abstract
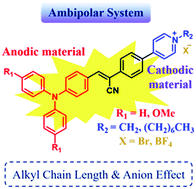
A series of aggregation induced emission (AIE)-active and redox-active α-cyanostilbene-containing triphenylamine derivatives with different alkyl chain lengths and anions were successfully synthesized, and their optical, photoluminescent and electrochromic behaviors were investigated. In addition, the intrinsically ambipolar system was generated by incorporating the pyridinium moiety, which serves as a charge storage unit to effectively balance the charge during the electrochemical oxidation redox process. The photoluminescent performance of the resulting AIE- and redox-active materials in solution, aggregated and solid states was influenced by the different alkyl chains as well as anions.


 Please wait while we load your content...
Please wait while we load your content...