ALD-based hydrothermal facile synthesis of a dense WO3@TiO2–Fe2O3 nanodendrite array with enhanced photoelectrochemical properties†
Abstract
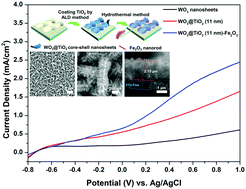
Hierarchical heterostructured photoanodes have been considered as ideal candidates for oxidation in an effort to enhance the photoelectrochemical (PEC) performance. In this study, well-defined WO3@TiO2–Fe2O3 nanodendrite arrays composed of WO3@TiO2 core–shell nanosheets and Fe2O3 nanorods are synthesized by atomic layer deposition and a subsequent hydrothermal process. The growth of Fe2O3 nanorods on the surface of WO3@TiO2 is demonstrated to be an effective strategy to improve the active surface area and charge separation, which have been proved by the band structure analysis and electrochemical characterization. Particularly, the photocurrent density of the WO3@TiO2–Fe2O3 nanodendrite array with an 11 nm-thick TiO2 buffer layer (∼1.92 mA cm−2) is much higher than the photocurrent density of the WO3 photoanode (∼0.41 mA cm−2) at 1.23 V vs. RHE in 0.5 M Na2SO4 aqueous solutions (pH = 6.8), with outstanding long-term stability as well. This remarkable improvement that comes from rational compositional and structural design of the WO3@TiO2–Fe2O3 nanodendrite array may provide much inspiration for the development of heterostructured PEC electrodes and devices with novel properties.


 Please wait while we load your content...
Please wait while we load your content...