Terrylene diimide-based middle-low bandgap electron acceptors for organic photovoltaics†
Abstract
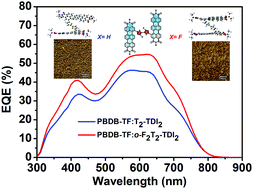
Rylene diimides have evolved as the most investigated compounds among polycyclic aromatic hydrocarbons, due to their excellent absorption, fluorescence and outstanding electron-withdrawing ability. In this work, we present two A–D–A type electron acceptors based on terrylene diimides and investigate the impact of intramolecular nonbonding conformational locks on the molecular geometry, the solid packing arrangement and the photovoltaic performance. Detailed investigation demonstrates that the introduction of fluoride atoms facilitates the noncovalent interactions with sulfur elements on adjacent thiophene groups and therefore, more balanced charge transfer and suppressed bimolecular recombination promotes the JSC and FF, endowing the solar cells based on fluoride-substituted acceptors with a higher PCE of up to 5.29%.


 Please wait while we load your content...
Please wait while we load your content...