A multistimuli responsive heteroleptic iridium(iii) complex: role of hydrogen bonding in probing solvent, pH and bovine serum albumin (BSA)†
Abstract
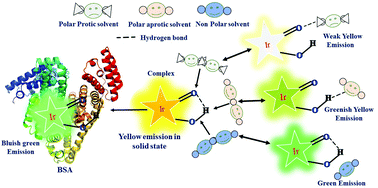
This article focuses on the vital role of hydrogen bonding to explain some unusual photophysical behaviors of an ‘Aggregation-induced emission’ (AIE) active Iridium(III) complex. The preponderance of hydrogen bonding leads to the complex's multifunctional character, viz., sensing ability of base and protein (BSA), pH probing, and solvatochromism. Depending on the hydrogen bonding capacity of the solvents, the emission properties of the complex are changed, i.e., green emission in non-polar solvents, yellowish-green emission in a chlorinated solvent, bright yellow emission in a polar aprotic solvent and weak yellow emission in polar protic solvent. A robust green emission was obtained with the addition of BSA into a solution of 1. The sensitivity of the complex to BSA was measured to be 9.3 pM. The mechanisms in all these cases have been explored based on control experiments and computational calculations/studies (DFT, TD-DFT, Docking).


 Please wait while we load your content...
Please wait while we load your content...