Modulation of π-linkers in asymmetric thermally activated delayed fluorescence molecules enabling high performance OLEDs†
Abstract
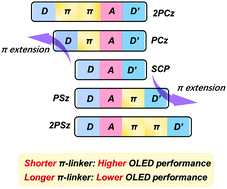
The role of aromatic π bridges (called π-linkers), which are used to connect multiple D and A groups in asymmetric thermally activated delayed fluorescence (TADF) emitters, remains a critical issue for the development of high-efficiency organic light-emitting diodes (OLEDs). In this work, based on our previous asymmetric TADF emitter (SCP) with electron donors carbazole (D) and phenothiazine (D′), we introduced different numbers of phenyl rings as π-linkers on both sides of SCP, leading to four D–A–D′ TADF emitters (PCz, 2PCz, PSz, and 2PSz) with different levels of conjugation. Experimental and theoretical results reveal that the π-linker distance has a significant effect on excited state regulation and thereby the optoelectronic properties. In comparison, the TADF emitters (PCz, and PSz) with shorter π-linkers exhibit higher photoluminescence quantum yields, due to effective suppression of conformation changes between ground state and excited state, and consequently higher device performance with external quantum efficiencies (EQEs) ranging from 20% to 30% achieved. This work provides an insight into the relationship between molecular design and optoelectronic properties of asymmetric TADF emitters.


 Please wait while we load your content...
Please wait while we load your content...