Effects of fluorine substitution in quinoidal oligothiophenes for use as organic semiconductors†
Abstract
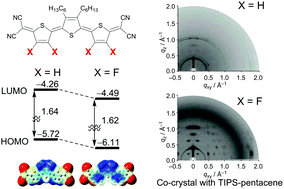
Tuning the electronic properties of π-conjugated molecules is an important subject in the development of organic semiconducting materials. Fluorine substitution on π-conjugated frameworks is recognized as a rational molecular design for lowering the molecular orbital energies. However, the development of quinoidal oligothiophenes to accomplish this strategy has lagged behind. Here, we report on a systematic study of bis(dicyanomethylene)-substituted quinoidal oligothiophene derivatives bearing fluorine atoms to clarify the influence of fluorine substitution on the structure, properties, and semiconducting characteristics of such compounds. Experimental and theoretical studies revealed that intramolecular S–F non-covalent interactions occur in these molecules, leading to the formation of fixed anti-conformations. Electrochemical measurements show that these compounds have increased electron-accepting characteristics compared to the corresponding non-fluorinated quinoidal molecules. X-ray diffraction measurements indicate that thin films that are made up of these molecules possess a high level of crystallinity. These properties lead to the appearance of n-type conduction in organic field-effect transistors (OFETs), even under ambient conditions. Furthermore, blends of these fluorine substituted compounds with p-type pentacene show good ambipolar behavior in OFETs. These results demonstrate that fluorine-substituted quinoidal oligothiophenes have broad potential for use in organic semiconducting materials.


 Please wait while we load your content...
Please wait while we load your content...