The effect of a vitamin B12 based catalyst on hydrogen peroxide oxidation reactions and the performance evaluation of a membraneless hydrogen peroxide fuel cell under physiological pH conditions†
Abstract
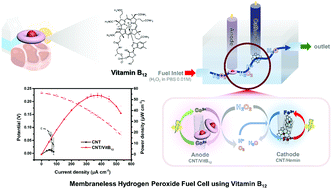
A new anodic catalyst containing carbon nanotubes (CNTs) and vitamin B12 (CNT/VitB12) and its electrochemical activation are introduced for promoting the hydrogen peroxide oxidation reaction (HPOR) and the performance of a membraneless hydrogen peroxide fuel cell (HPFC). Through activation, the anodic current density of the activated catalyst (CNT/VitB12-Co2+) (271.0 μA cm−2) is 2.28 times better than that of the non-activated catalyst (CNT/VitB12-Co3+) (118.8 μA cm−2) at a low potential (0.4 V) when only 3 mM H2O2 is injected. This is due to the two-step HPOR mechanism of CNT/VitB12 where Co2+ ions are initially converted into Co3+ ions by the reaction with H2O2 (step 1) and electrons are generated when Co3+ ions return to Co2+ ions (step 2). Such produced electrons play a role in increasing the anodic current density. Based on that, it is confirmed that electrochemical activation controlling the oxidation state of Co ions is a key factor to enhance the catalytic activity of CNT/VitB12. Regarding stability, desirable CNT/VitB12-Co2+ preserves its initial current for 2 h in an amperometric response test. The onset potentials for CNT/Vit B12 and CNT/hemin, which are used as cathodic catalysts, are 0.1 and 0.35 V vs. Ag/AgCl, respectively, indicating that when the two catalysts and H2O2 fuel are used, the open circuit voltage (OCV) is 0.25 V. When the membraneless HPFC is operated, the OCV and maximum power density are 0.233 ± 0.005 V and 53.8 ± 0.4 μW cm−2 with 0.1 M H2O2 fuel. The similarity between the difference in onset potential of the catalysts and the OCV of the membraneless HPFC is evidence that the catalytic activity of the catalysts is the dominant parameter to determine the performance of membraneless HPFCs. The suggested membraneless HPFC shows an excellent performance despite a low fuel concentration and membraneless structure.


 Please wait while we load your content...
Please wait while we load your content...