Subnaphthalocyanine triimides: potential three-dimensional solution processable acceptors for organic solar cells†
Abstract
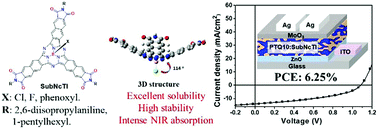
Subnaphthalocyanine triimides (SubNcTIs) as solution processable electron acceptors were designed and synthesized by introducing three electron-withdrawing imide groups to subnaphthalocyanines. Their solubility and crystallinity could be adjusted conveniently by substituents at imide terminals or boron atoms. Their absorption, electrochemistry, thermal properties, and applications as electron acceptors in bulk heterojunction organic solar cells (BHJOSCs) were investigated. SubNcTIs with strong absorption at 300–750 nm, maximum extinction coefficient of up to 16.8 × 104 M−1 cm−1, and deep lowest unoccupied molecular orbital energy levels (−3.79 to −3.90 eV) are expected to be excellent electron acceptors. The four SubNcTIs exhibit good thermal stability, with 5% weight loss at a temperature higher than 350 °C. Blending with the donor polymer of PTQ10, BHJOSCs based on acceptor 9b gave the highest power conversion efficiency (PCE) of 6.25%, which is the highest value among the solution processable cyanine family. Space-charge-limited current (SCLC), charge recombination, and charge collection ability measurements showed that PTQ10:9b devices have a high and balanced carrier mobility, less charge recombination, and better charge transport, which lead to high photovoltaic performance. Grazing incidence wide-angle X-ray scattering (GIWAXS) measurements revealed that relatively strong π–π stacking and large correlation lengths of PTQ10:9b films are favorable for charge transfer, which caused a high Jsc of corresponding solar cells. This study demonstrates that SubNcTIs as a promising chromophore could be used to construct potential electron acceptors.


 Please wait while we load your content...
Please wait while we load your content...