Bisindole caulerpin analogues as nature-inspired photoresponsive molecules†
Abstract
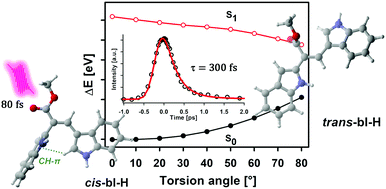
Photoresponsive molecules are present in many organisms, including animals and plants. These kinds of pigments absorb UV or visible radiation and efficiently dissipate the photon energy through pathways that involve intramolecular rearrangements. In this work, a set of bisindole analogs of the natural pigment caulerpin were synthesized and evaluated as photoresponsive systems. The bisindoles of this study are modified versions of the caulerpin metabolite which conserve an asymmetric methinic bridge between two indolyl moieties. In the ground state the more stable conformation for these systems was found to be the E-isomer. Detailed NMR experiments show that upon UVA irradiation (400 nm) the bisindoles evolve to the highly stable Z-isomer, which is persistent in solution for several weeks in the dark. Femtosecond resolved measurements revealed a 300 fs deactivation channel for the first singlet excited state which is associated with evolution in the S1 states leading to ultrafast E–Z photoisomerization and a return to the electronic ground state. The excited-state dynamics were also characterized by mapping changes in the electron density in the first singlet excited state. This study shows evident charge density migration away from the double-bond region upon irradiation. The redistribution of the electron density is followed by pyramidalization of the adjacent carbon atoms while torsion takes place. The synthesized bisindolic core of this study can lead to the development of novel and easy to functionalize photoresistant and photoresponsive molecules, potentially applicable as photo-controllable molecular switches.


 Please wait while we load your content...
Please wait while we load your content...