The construction of helicate metal–organic nanotubes and enantioselective recognition†
Abstract
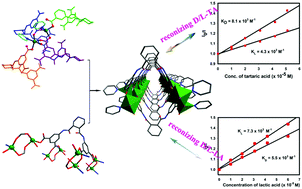
Intriguing 1D single-walled metal–organic nanotubes (MONTs) Zn2(RR-CHCAIP)Py2(H2O) (HMOF-2) were successfully self-assembled using a semirigid homochiral ligand, 5,5′-((1R,2R)-cyclohexane dicarbonyl bis(azanediyl)) diisophthalic acid (RR-CHCAIP) with zinc salt, which was constructed from an organic ligand similar to the twin blades of a propeller with the Zn2(COO)4(H2O)(Py)2 cluster acted as the SBU, and the terminal pyridine auxiliary ligand capping at the Zn atoms played a key role in the formation of the 1D single nanotubes. The fluorescence of the HMOF-2 emulsion could be effectively quenched by D/L-lactic acid, D/L-tartaric acid enantiomers and D/L-alanine via hydrogen bonding interactions with the inwall of MONTs, which suggested this to be a remarkable chiral sensor for α-hydroxyl carboxylic acid and amino acid enantiomers with good sensitivity and enantioselectivity. Comparatively, HMOF-2 showed a negligible response towards D/L-mandelic acid and D/L-tyrosine, which chiefly arose from the confinement effect and the host–guest interaction of MONTs with the analytes. The construction strategy of MONTs based on the homochiral propeller-like ligand that bridges two metallic pillars will open new perspectives for developing self-assembling chiral MONTs with unique and practically useful enantioselective functions.


 Please wait while we load your content...
Please wait while we load your content...