Nickel sulfide nanocrystals for electrochemical and photoelectrochemical hydrogen generation†
Abstract
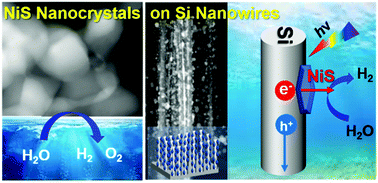
Photoelectrochemical water splitting has been considered as the most promising technology for generating hydrogen energy. Herein, we report nickel sulfide nanocrystals (NCs) as excellent catalysts for electrochemical and solar-driven photoelectrochemical (PEC) hydrogen evolution. Two polymorphic NiS and NiS1.97 NCs were synthesized with a controlled composition, and the NiS NCs showed higher electrocatalytic activity than the NiS1.97 NCs toward the hydrogen/oxygen evolution reactions in 0.5 M H2SO4 and 1 M KOH. Photocathodes were fabricated by growing the NiS and NiS1.97 NCs directly onto Si nanowire (NW) arrays. The deposition of NiS NCs results in excellent PEC performance in both acid and alkaline electroytes; the photocurrent is 10 mA cm−2 (at 0 V vs. RHE) and the onset potential is 0.2 V. In contrast, the Si-NiS1.97 photocathode exhibits much lower photocurrent and less stability. Detailed structure analysis using X-ray photoelectron spectroscopy reveals that the NiS is more metallic than the NiS1.97 and retains the same electronic structures during the catalytic reaction. The high catalytic activity of metallic NiS NCs should be the main cause of this enhanced PEC performance, promising efficient water-splitting Si-based PEC cells.


 Please wait while we load your content...
Please wait while we load your content...