In situ planar photoalignment of liquid crystals: two-step interfacial modifications through light–matter interactions actuated by linearly polarized UV-light†
Abstract
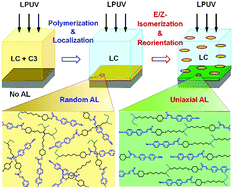
Photopolymerizable, photoisomerizable, photodimerizable and photoluminescent dichroic chromophores are deliberately designed and used for comprehending the sequential realization of the polyimide-free in situ photoalignment of liquid crystals (LCs). Upon a linearly polarized UV-light (LPUV) treatment, the multifunctional photoaligning additive, homogeneously dissolved in a LC mixture, is interfacially polymerized at the inner surfaces as a thin photo-responsive polymer layer. Subsequently, the molecular orientational anisotropy is induced by the dichroic photochromic responses, resulting in a uniaxial planar LC alignment. The reversible trans/cis-isomerization plays a crucial role for the LC alignment, rather than irreversible [2+2] dimerization. Therefore, the LC aligning effect is strongly influenced by the wavelengths of LPUV. Under the longer wavelength UV-irradiation (λ > 350 nm), the reversible isomerization is more preferable to the irreversible dimerization, resulting in a rewritable LC alignment with much better alignment quality. Such sequential processes are evidenced by monitoring spatial and orientational distributions of the self-labeled dichroic fluorescent monomers during the process. The conclusions are further corroborated by the wavelength dependencies of the photochromic responses, LC aligning effect, and rewritable LC alignment.


 Please wait while we load your content...
Please wait while we load your content...