Stimuli-responsive phenothiazine-based donor–acceptor isomers: AIE, mechanochromism and polymorphism†
Abstract
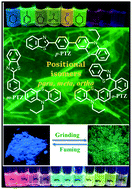
The development of solid-state stimuli responsive materials has escalated following their intriguing colour switching properties and versatile material and optoelectronic applications. In this article, we have designed and synthesized donor–acceptor (D–A) isomers, namely p-PTZ, m-PTZ and o-PTZ, by functionalizing the para, meta and ortho positions of the benzothiazole (BT) ring with the phenothiazine (PTZ) moiety. The Suzuki cross-coupling reaction of the mono boronate ester of phenothiazine PTZ with the corresponding bromo derivatives of phenyl BT generated the isomers p-PTZ, m-PTZ and o-PTZ. The attachment of donor PTZ at different positions of the acceptor phenyl BT could exert influence on the donor–acceptor character and the molecular packing modes in the isomers, resulting in discrete photophysical and electronic properties. The isomers manifest contrasting emission properties in different solvents as a consequence of the twisted intramolecular charge transfer (TICT) state. The non-planar twisted structures of the isomers bring about aggregation induced emission (AIE) characteristics and reversible mechanofluorochromism (MFC) behaviour. The isomers display self-reversible colour switching by virtue of the different accessible conformers of the PTZ moiety. The p-PTZ isomer occurs as polymorphs owing to the conformational flexibility of the PTZ moiety. The structural and morphological differences in the polymorphs of p-PTZ were studied in detail using single crystal X-ray analysis, scanning electron microscopy (SEM) and powder X-ray diffraction (PXRD) studies. Density functional theory (DFT) and time-dependent density functional theory (TDDFT) calculations were used to explore the electronic properties of the isomers in the ground and excited state. The isomers were capable of sensing trifluoroacetic acid in solution. A detailed comparison of the photophysical, electronic and structural properties of p-PTZ, m-PTZ and o-PTZ has been done with a view to studying the outcome of the positional change. The adopted design opens up different possibilities for study of novel stimuli responsive materials.


 Please wait while we load your content...
Please wait while we load your content...