Abstract
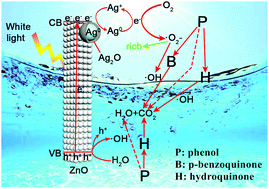
Photocatalysis, as a potential green technology, can effectively degrade organic pollutants. The changes of the intermediate products in the photocatalytic degradation process of pollutants are particularly important for studying the degradation mechanism. In the present paper, ZnO porous nanorod (NR), ZnO/Ag and ZnO/Ag/Ag2O photocatalysts were prepared and their photocatalytic phenol degradation performance and processes were investigated. ZnO/Ag/Ag2O displays a significantly enhanced photocatalytic phenol degradation performance with complete degradation of phenol in only 90 min of white light illumination. Except for the direct ring-opening reaction of phenol, hydroquinone is the main intermediate product for the ZnO NRs and ZnO/Ag in the photocatalytic phenol degradation process. However, for the ZnO/Ag/Ag2O photocatalyst, the Ag/Ag2O nanoparticles (NPs) act as acceptors of the photogenerated electrons and play an important role in the phenol degradation process. Different from the ZnO NRs and ZnO/Ag, ZnO/Ag/Ag2O can selectively catalyze the formation of the p-benzoquinone intermediate due to the generation of a large amount of ˙O2− through the mutual conversion of Ag0 and Ag+ on the Ag/Ag2O NPs. Therefore, ZnO/Ag/Ag2O accelerates the oxidation and degradation of phenol to carbon dioxide and water through different intermediate processes under white light illumination.
- This article is part of the themed collection: Journal of Materials Chemistry C Lunar New Year collection 2021


 Please wait while we load your content...
Please wait while we load your content...