Biomedical application of mesoporous silica nanoparticles as delivery systems: a biological safety perspective
Abstract
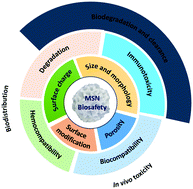
The application of mesoporous silica nanoparticles (MSNs) as drug delivery systems to deliver drugs, proteins, and genes has expanded considerably in recent years, using in vitro and animal studies. For future translation to clinical applications, the biological safety aspects of MSNs must be considered carefully. This paper reviews the biosafety of MSNs, examining key issues such as biocompatibility, effects on immune cells and erythrocytes, biodistribution, biodegradation and clearance, and how these vary depending on the effects of the physical and chemical properties of MSNs such as particle size, porosity, morphology, surface charge, and chemical modifications. The future use of MSNs as a delivery system must extend beyond what has been learnt thus far using rodent animal models to encompass larger animals.


 Please wait while we load your content...
Please wait while we load your content...