The application of nitrogen heterocycles in mitochondrial-targeting fluorescent markers with neutral skeletons†
Abstract
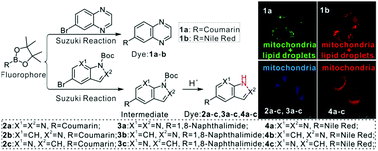
Four different neutral fluorescent markers containing nitrogen heterocycles (quinoxaline, 1H-pyrazolo[3,4-b]pyridine, 1H-indazole and 1H-pyrrolo[2,3-b]pyridine) as targeting groups were designed and prepared in order to screen out structural units for targeting mitochondria. Several classical fluorophores (coumarin, 1,8-naphthalimide and Nile Red) were connected with these heterocycles via Suzuki coupling reactions. The derivatives of coumarin (dyes 1a and 2a–c) and 1,8-naphthalimide (dyes 3a–c) fluoresced in the blue-green region, while the Nile Red derivatives (dyes 1b and 4a–c) fluoresced in the red light region. The optical properties of the classical fluorophores, such as emission properties and photostability, were retained in the new dyes. All of them showed low cytotoxicity. Confocal fluorescence experiments in L929 normal cells and HeLa cancer cells indicated that dyes 1a–b targeted dual sites of mitochondria and lipid droplets. Moreover, dyes 2a–c, 3a–c and 4a–c targeted mitochondria; meanwhile, there are only a few mitochondria-targeting markers with neutral skeletons. Furthermore, it was found that nitrogen heterocycles with N–H bonds can improve the mitochondrial targeting ability of partial neutral fluorophores.


 Please wait while we load your content...
Please wait while we load your content...