Unraveling the mechanism for an amelogenin-derived peptide regulated hydroxyapatite mineralization via specific functional domain identification†
Abstract
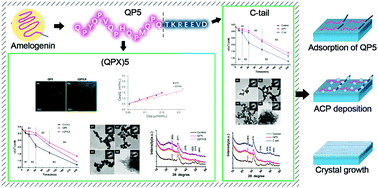
Amelogenin and its various derived peptides play important roles in promoting biomimetic mineralization of enamel. Previously, an amelogenin-derived peptide named QP5 was proved to be able to repair demineralized enamel. The objective here was to interpret the mechanism of QP5 by elucidating the specific function of each domain for further sequence and efficacy improvement. Peptide QP5 was separated into domains (QPX)5 and C-tail. (QPX)3 was also synthesized to investigate how QPX repeats affect the mineralization process. Circular dichroism spectroscopy showed that two (QPX) repeats adopted a β-sheet structure, while C-tail exhibited a disordered structure. (QPX)5 showed more absorption in confocal laser scanning microscopy observation and a higher K value in Langmuir adsorption isotherms compared to C-tail, while (QPX)3 with better hydropathy had greater adsorption capability than (QPX)5. Meanwhile, calcium consumption kinetics, transmission electron microscopy and selected area electron diffraction indicated that (QPX)5, C-tail and (QPX)3 had similar inhibitory effects on the spontaneous calcium consumption and the morphology of their nucleation products were alike, while QP5 had a greater inhibitory effect than them and induced elongated plate-like crystals. X-Ray diffraction further showed that both C-tail and (QPX)3 had greater potential in improving the apatite crystal orientation degree. In conclusion, (QPX)5 was the major adsorption region, both (QPX)5 and C-tail inhibited the nucleation, and C-tail contributed more to improve the HAP orientation degree, so QP5 could exert a significant remineralization effect. By reducing two repeats, (QPX)3 showed higher hydropathicity than (QPX)5 and achieved higher binding affinity, and it was more potential in improving the HAP orientation degree with lower economic cost.


 Please wait while we load your content...
Please wait while we load your content...