Controlled release of small molecules and proteins from DNA-surfactant stabilized metal organic frameworks†
Abstract
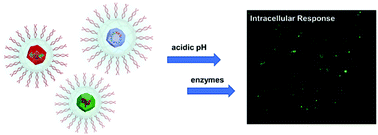
This work highlights a multifunctional nanoscale material which can effectively compartmentalize small molecules and biomolecules into a single, micellar structure with programmable degradation properties resulting in highly controllable release properties. The nanomaterial consists of a ZIF-8 metal organic framework (MOF) encapsulated within a DNA surfactant micelle assembly, referred to as a nucleic acid nanocapsule (NAN). NANs have been demonstrated to enter cells through endocytosis and result in intracellular cargo release upon enzyme-triggered degradation. By combining the favorable properties of MOFs (large storage capacity) with those of NANs (triggerable release), we show diverse molecular cargo can be integrated into a single, highly programmable nanomaterial with controllable release profiles. The hybrid MOF–NANs exhibit double-gated regulation capabilities as evidenced by kinetic studies of encapsulated enzymes that indicate individual layers of the particle influence the overall enzymatic rate of turnover. The degradation of MOF–NANs can be controlled under multiple combined stimuli (i.e. varying pH, enzymes), enabling selective release profiles in solutions representative of more complex biological systems. Lastly, the enhanced control over the release of small molecules, proteins and plasmids, is evaluated through a combination of cell culture and in vitro fluorescence assays, indicating the potential of MOF–NANs for both therapeutic and diagnostic applications.


 Please wait while we load your content...
Please wait while we load your content...