Coordination-driven reversible surfaces with site-specifically immobilized nanobody for dynamic cancer cell capture and release†
Abstract
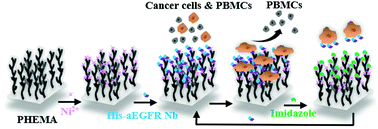
Selective isolation of circulating tumor cells (CTCs) from blood provides a non-invasive avenue for the diagnosis, prognosis and personalized treatment for patients with cancer. The specific capture of CTCs is conventionally based on the immunoaffinity recognition between antibody and receptor on cell membranes. However, using a traditional antibody for high-efficiency isolation of CTCs remains a challenge due to the limited loading capacity of the large antibodies on material surfaces. Herein, using a small-sized nanobody (Nb), we developed a widely applicable strategy to construct reversible site-specifically immobilized Nb surfaces for the capture and release of epidermoid cancer cell line A431 cells. Coordination interaction between the histidine tag (His-tag) of the nanobody (Nb) and Ni2+ ions that chelated to the NTA-modified poly(2-hydroxyethyl methacrylate) (PHEMA) brushes was used to achieve site-specific immobilization of EGFR Nb (PHEMA-aEGFR surfaces). The high-density immobilized nanobody possessing maximized activity resulted in the high-efficiency capture of 81% rare A431 cells within just 30 min, showing a higher capture yield and shorter capture time compared with that achieved by the conventional antibody immobilized on the flat surface. Additionally, the PHEMA-aEGFR surfaces exhibited low capture limit (1 cell mL−1), cytocompatibility for captured cells, as well as negligible non-specific adhesion of PBMCs. With a one-step treatment using imidazole for competitive coordination, 86% of the captured cells were effectively released. This multifunctional and dynamic site-specifically immobilized nanobody strategy paves a new path in the development of materials and instruments for the high-efficiency capture and release of rare cells at a low cost.


 Please wait while we load your content...
Please wait while we load your content...