Spatiotemporal neocartilage growth in matrix-metalloproteinase-sensitive poly(ethylene glycol) hydrogels under dynamic compressive loading: an experimental and computational approach
Abstract
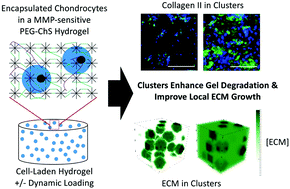
Enzyme-sensitive hydrogels containing encapsulated chondrocytes are a promising platform for cartilage tissue engineering. However, the growth of neotissue is closely coupled to the degradation of the hydrogel and is further complicated due to the encapsulated cells serving as the enzyme source for hydrogel degradation. To better understand these coupled processes, this study combined experimental and computational methods to analyze the transition from hydrogel to neotissue in a biomimetic MMP-sensitive poly(ethylene glycol) (PEG) hydrogel with encapsulated chondrocytes. A physics-based computational model that describes spatial heterogeneities in cell distribution was used. Experimentally, cell-laden hydrogels were cultured for six weeks under free swelling or subjected daily to one-hour of dynamic compressive loading. Extracellular matrix (ECM) synthesis rates were used as model inputs, and the model was fit to the experimentally determined construct modulus over time for the free swelling condition. Experimentally, ECM accumulation comprising collagen II and aggrecan increased over time concomitant with hydrogel degradation observed by a loss in PEG. Simulations demonstrated rapid degradation in regions of high cell density (i.e., cell clusters) reaching complete degradation by day 13, which facilitated localized ECM growth. Regions of low cell density degraded more slowly, had limited ECM, and led to the decrease in construct modulus during the first two weeks. The primary difference between the two culture environments was greater ECM accumulation in the clusters under free swelling, which facilitated a faster recovery in construct modulus. By 6 weeks the compressive modulus increased 2.5-fold to 107 kPa under free swelling, but dropped 1.6-fold to 26 kPa under loading. In summary, this biomimetic MMP-sensitive hydrogel supports neocartilage growth by facilitating rapid ECM growth within cell clusters, which was followed by slower growth in the rest of the hydrogel. Subtle temporal differences in hydrogel degradation and ECM accumulation, however, had a significant impact on the evolving mechanical properties.


 Please wait while we load your content...
Please wait while we load your content...