Facilitating Myers–Saito cyclization through acid-triggered tautomerization for the development of maleimide-based antitumor agents†
Abstract
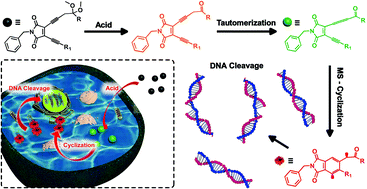
Enyne-allene compounds undergo Myers–Saito cyclization at physiological temperature to generate diradical intermediates that are capable of inducing DNA damage and cell death. The high reactivity of enyne-allene however limits their promising prospect as anticancer agents due to the spontaneous cyclization during storage and delivery. Regulating the cyclization process by taking advantage of the characteristics of a tumor cellular microenvironment, such as employing a low pH value to activate the cyclization process, is thus of essential importance. In this work, a novel enediyne (EDY) system with locked carbonyl groups was specifically designed and synthesized. Unlocking the protected carbonyl groups in the presence of acid would facilitate the rearrangement of propargyl moieties into an allene group, enabling the formation of an enyne-allene structure and occurrence of Myers–Saito cyclization. The pH-dependent diradical generation and DNA-cleavage ability of the designed EDY system were confirmed by electron paramagnetic resonance analysis and DNA gel electrophoresis. A promising cytotoxicity against HeLa cells with half inhibition concentrations (IC50) as low as 1.40 μM was obtained, which was comparable to those of many commercially applied anticancer drugs. Further in vitro experiments revealed that this EDY system induced intracellular DNA damage and subsequently resulted in S-phase arrest and cytotoxicity through programmed apoptosis.


 Please wait while we load your content...
Please wait while we load your content...