Rituximab conjugated iron oxide nanoparticles for targeted imaging and enhanced treatment against CD20-positive lymphoma†
Abstract
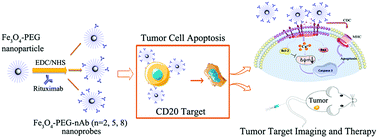
Since its launch in 1997, rituximab (RTX) has extensively improved the treatment of CD20-positive follicular and diffuse large B cell non-Hodgkin lymphoma (NHL). The application of RTX is limited usually by the failed therapy because of resistance. Iron oxide nanomaterials have been explored for cancer detection and treatment in recent years. In this study, a multivalent nanoprobe comprising one Fe3O4 nanoparticle and several RTX antibodies was constructed for the targeted imaging and enhanced treatment of NHL. Poly(ethylene glycol) (PEG)-coated Fe3O4 nanoparticles were fabricated via a thermal decomposition method and ligand exchange. RTX was conjugated onto the surface of the Fe3O4-PEG nanoparticles to form Fe3O4-PEG-nAb (n = 2, 5 or 8) multivalent nanoprobes. These multivalent nanoprobes, with a core size of approximately 11 nm and a hydrodynamic diameter of about 22 nm, showed colloidal stability in buffer solution. The r2 relaxation rate of Fe3O4-PEG-nAb was similar to that of Fe3O4-PEG (309 ± 3.08 mM−1 s−1). The specificity of nanoprobes for CD20-positive Raji cells was assessed on a clinical magnetic resonance imaging scanner. The receptor binding site of one multivalent nanoprobe was more than that of one RTX, exhibiting valence-dependent induction of Raji cell apoptosis, and this effect could be enhanced by complement activation from blood serum added. A similar activity was observed in vivo in a NHL xenograft model. The multivalent nanoprobe treatment significantly reduced tumor burden and enhanced survival in comparison to the RTX group. Our studies demonstrate that the appropriate design and preparation of anticancer antibody–nanoparticle conjugates enable the generation of improved anticancer nanomedicines and could thus provide an efficient cancer theranostic strategy.


 Please wait while we load your content...
Please wait while we load your content...