Amphiphilic phthalocyanines in polymeric micelles: a supramolecular approach toward efficient third-generation photosensitizers†
Abstract
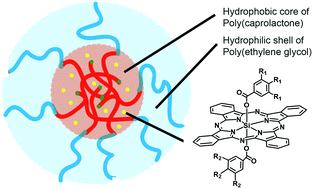
In this paper we describe a straightforward supramolecular strategy to encapsulate silicon phthalocyanine (SiPc) photosensitizers (PS) in polymeric micelles made of poly(ε-caprolactone)-b-methoxypoly(ethylene glycol) (PCL–PEG) block copolymers. While PCL–PEG micelles are promising nanocarriers based on their biocompatibility and biodegradability, the design of our new PS favors their encapsulation. In particular, they combine two axial benzoyl substituents, each of them carrying either three hydrophilic methoxy(triethylenoxy) chains (1), three hydrophobic dodecyloxy chains (3), or both kinds of chains (2). The SiPc derivatives 1 and 2 are therefore amphiphilic, with the SiPc unit contributing to the hydrophobic core, while lipophilicity increases along the series, making it possible to correlate the loading efficacy in PCL–PEG micelles with the hydrophobic/hydrophilic balance of the PS structure. This has led to a new kind of third-generation nano-PS that efficiently photogenerates 1O2, while preliminary in vitro experiments demonstrate an excellent cellular uptake and a promising PDT activity.


 Please wait while we load your content...
Please wait while we load your content...