Enhanced bactericidal activity of brucite through partial copper substitution†
Abstract
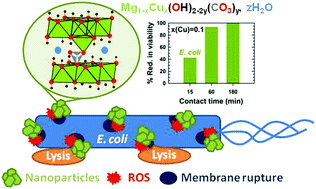
Brucite Mg(OH)2 belongs to a family of two-dimensional compounds with a CdI2-type structure built up from layers of edge-sharing octahedra delineating 2D galleries. In the current study, nanometer-sized platelets of copper substituted Mg(OH)2 were prepared by co-precipitation at room temperature in mixed alkaline (NaOH/Na2CO3) medium. Very weak substitution of a few hydroxyl ions by carbonate groups was highlighted at first by infrared spectroscopy and then quantified by thermogravimetric (TG) and mass spectrometric (MS) evolved gas analyses. The presence in a very low amount of water molecules in the galleries induces disorder in the stacking of layers of edge-sharing octahedra along the c-axis. The dehydration of the hydroxides taking place below 225 °C preserves the brucite-type structure of the samples while suppressing the stacking disorder. Copper substitution greatly enhances the bactericidal activity of nanometer-sized platelets of brucite against two bacteria frequently involved in healthcare-associated-infections. 10 mol% of cupric ions in Mg(OH)2 (a copper loading of 0.102 mg mL−1 in the suspension) were sufficient to induce, after 3 h in contact, 100% and 99.3% reductions in viability of Gram-negative E. coli and Gram-positive S. aureus, respectively (reductions as low as 23% and 48% are reported for the parent compound Mg(OH)2 in the same conditions). A good compromise between fast bactericidal kinetics and a high reduction in viability is reached by the 15 mol% copper-substituted Mg(OH)2 hydroxide. Its use gives the opportunity to five-fold reduce the copper loading of the bactericidal agent while being at least equally or even more efficient compared to the conventional CuO (a Cu loading of 0.799 mg mL−1 and 0.154 mg mL−1 in the suspension of CuO and 15 mol% copper substituted Mg(OH)2 particles, respectively).


 Please wait while we load your content...
Please wait while we load your content...