Cogeneration of ethylene and electricity in symmetrical protonic solid oxide fuel cells based on a La0.6Sr0.4Fe0.8Nb0.1Cu0.1O3−δ electrode†
Abstract
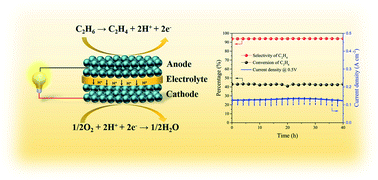
La0.6Sr0.4Fe0.9Nb0.1O3−δ (LSFN) and La0.6Sr0.4Fe0.8Nb0.1Cu0.1O3−δ (LSFNCu) perovskite oxides are prepared as catalysts for C2H6 dehydrogenation. The partial substitution of Fe by Cu enhances the structural stability and significantly increases the formation of oxygen vacancies and also the oxidation states of the Fe cations. The performances of the catalysts are investigated from 650 to 750 °C in a fixed-bed reactor. The C2H6 conversion by LSFNCu is about 42% at 750 °C, which is obviously higher than that by LSFN (37.8%). Furthermore, a symmetrical protonic solid oxide fuel cell reactor with the configuration of LSFNCu/BZCYYb/LSFNCu is prepared to cogenerate ethylene and electricity from ethane. At 750 °C, the peak power density of the symmetrical cell reaches 90 mW cm−2 in C2H6. Meanwhile, C2H6 conversion is about 41.5% at OCV conditions and can be increased to 43.4% under a constant current of 200 mA cm−2. This indicates that the consumption of the formed H2 promotes the dehydrogenation reactions. The performance of the fuel cell reactor is essentially stable during the 40 h test, confirming that LSFNCu is an excellent coking resistant electrocatalyst for cogeneration of C2H4 and electricity from ethane.


 Please wait while we load your content...
Please wait while we load your content...