Dynamic evolution of isolated Ru–FeP atomic interface sites for promoting the electrochemical hydrogen evolution reaction†
Abstract
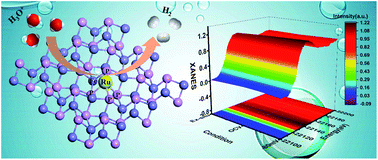
Identification of active sites in the electrochemical hydrogen evolution reaction (HER) under realistic conditions is critical to water electrolysis for future sustainable energy applications. Herein, we rationally designed an isolated single-atom Ru-modified FeP as a model catalyst with enhanced HER performance, which displayed an overpotential of 62 mV at a current density of 10 mA cm−2 and a small Tafel slope of 45 mV dec−1. Importantly, through operando X-ray absorption spectroscopy measurements together with density functional theory calculations, a synergistic effect was discovered between the monodispersed metal atoms and the phosphide substrate. We found that the bond-length-extended isolated Ru(+3)–P4–Fe species under catalytic conditions serve as the boosted active sites during the HER, leading to charge redistribution at the atomic interface of the catalyst, meanwhile improving the H adsorption and H2 desorption behavior, and further promoting the HER kinetics. This work gives new viewpoints for the exploration of highly active transition-metal-phosphide-based HER catalysts by refinement of the atomic and electronic structure.


 Please wait while we load your content...
Please wait while we load your content...