Iron-regulated NiPS for enhanced oxygen evolution efficiency†
Abstract
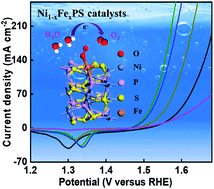
Development of robust and highly active electrocatalysts for the oxygen evolution reaction (OER) is of significance for next-generation renewable energy storage and conversion. Herein, for the first time, we report pyrite-type iron nickel monophosphosulfide (Ni1−xFexPS, x = 0, 0.1, 0.15, 0.2) electrocatalysts with exceptional OER efficiency and stability under alkaline conditions. Ni0.85Fe0.15PS/NF exhibits a considerably low overpotential of 251 (314) mV at 10 (100) mA cm−2 with a Tafel slope of 34 mV dec−1, together with remarkable stability. Moderate Fe regulation in NiPS (Ni0.85Fe0.15PS) is found to stimulate the activation of high-valence-state Ni/Fe oxyhydroxides during the irreversible surface reconstructions that form disordered MOOH@MxSOy@MxPOy surfaces under OER conditions, as is established by microstructural observations, surface-sensitive X-ray photoelectron spectroscopy, Raman spectroscopy and X-ray absorption spectroscopy. DFT calculations show that the catalytic sites formed by Fe doping are more nucleophilic than the Ni sites and thus more OER active, due to a facile Fe(II) → Fe(III) oxidation state change for Fe. Meanwhile, overly stable OER surface species are not formed, as Fe in oxidation states higher than Fe(III) is not favorable, which leads to a lower barrier for the rate-limiting OER step than that for the Ni site. The present results shed light on the design and development of high-performance electrocatalysts in ternary metal monophosphosulfides, as well as providing a fundamental understanding of their intrinsic active sites.


 Please wait while we load your content...
Please wait while we load your content...