In situ transformation of bismuth metal–organic frameworks for efficient selective electroreduction of CO2 to formate†
Abstract
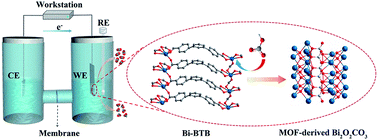
The bismuth-based materials are proven as valid electrocatalysts for electrochemical conversion of carbon dioxide (CO2) into formate (HCOO−). Herein, we discover that HCO3− can destroy the Bi–O bonds of the bismuth–carboxylate MOF to achieve in situ transformation into Bi2O2CO3 (MOF-derived Bi2O2CO3), which is merely explored yet. MOF-derived Bi2O2CO3 shows superior electrocatalytic performance in a broad range of potentials and demonstrates a formate faradaic efficiency (FEHCOO−) of 96.1% as well as an outstanding durability in 48 h with a current density about 10 mA cm−2 at a potential of −0.669 V vs. RHE. And compared with the structurally similar bismuth oxyhalides, MOF-derived Bi2O2CO3 possesses higher stability and lower CO2 concentration-dependence during CO2 reduction. Furthermore, this work puts a new strategy forward to achieve in situ MOF transformation and to comprehend the stability of electrocatalysts.


 Please wait while we load your content...
Please wait while we load your content...