Phase-controlled synthesis of Ni nanocrystals with high catalytic activity in 4-nitrophenol reduction†
Abstract
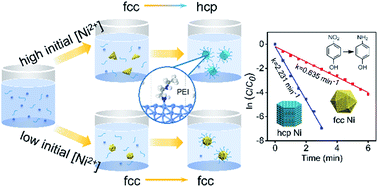
Controlled-synthesis of metal nanocrystals (NCs) with well-defined crystal phases has been developed as a promising strategy to tune their functional properties. However, the phase-controlled synthesis of Ni NCs remains a great challenge due to their thermodynamic instability. Here, we report a facile solvothermal method to prepare two typical well-defined Ni NCs, a hexagonal prism with a hexagonal close-packed (hcp) phase and an icosahedron with a face-centered cubic (fcc) phase. Their morphology and structure are well characterized, and the formation mechanism is systemically investigated. It is found that hcp Ni NCs are formed via an unusual phase transformation process from the thermodynamically stable fcc phase to metastable hcp phase. Both the experimental study and density functional theory (DFT) calculations reveal that the initial reduction rate of Ni2+ could control the internal structure of Ni at the nucleation stage, while the preferential adsorption of polyethylenimine (PEI) on hcp Ni facets plays a key role in the phase transformation and facet evolution of Ni NCs at the growth stage. Moreover, hcp Ni NCs exhibit superior catalytic activity and reusability in the reduction of 4-nitrophenol by NaBH4 with a remarkably higher reaction constant (2.23 min−1) than other non-noble metal catalysts (0.09–0.13 min−1) and some noble metal catalysts (0.14–1.96 min−1). This work provides a new approach for phase- and morphology-controlled synthesis of Ni NCs and new insight into their catalytic functions.


 Please wait while we load your content...
Please wait while we load your content...