What happens structurally and chemically during sodium uptake and release by Ni2P2S6: a combined X-ray diffraction, X-ray absorption, pair distribution function and MAS NMR analysis†
Abstract
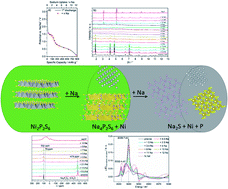
The layered compound Ni2P2S6 was electrochemically characterized for application as an anode material in sodium-ion batteries (SIBs). A high reversible capacity of 621 mA h g−1 at 1 A g−1 was achieved after 190 cycles. The investigation of the complex reaction mechanism of the conversion reaction was performed applying complementary techniques including X-ray powder diffraction, pair distribution function analysis, X-ray absorption spectroscopy, 19F/23Na/31P MAS NMR, TEM and nano-EDX. The results highlight that Na uptake for up to 5 Na per formula unit (f.u.) led to reduction of Ni2+ to metallic Ni nanoparticles and concomitant formation of an intermediate compound Na4P2S6. Increasing the Na content to 12 Na per f.u. generates nanocrystalline Na2S, which is accompanied by the loss of the long-range order of the pristine sample. In the completely discharged state elemental Ni and Na2S are present, but in contrast to literature reports, no evidence for the formation of NaxP phases was found. During the charge process, Ni3S2 is formed upon the release of ∼11.7 Na per f.u.


 Please wait while we load your content...
Please wait while we load your content...