Iron clusters boosted performance in electrocatalytic carbon dioxide conversion†
Abstract
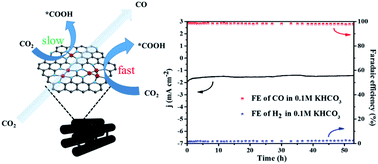
Metal clusters with exposed atomic interfaces and unique electronic structures have received considerable attention in heterogeneous catalysis. However, their potential application in the electrocatalytic CO2 reduction reaction (CO2RR) remains very challenging because metal clusters tend to drive the competitive hydrogen evolution reaction. In this study, we prepared Fe-cluster sites with positive valence anchored on ordered mesoporous carbon materials for use as an efficient and durable electrocatalyst in carbon dioxide electroreduction. The as-prepared catalyst exhibits high selectivity, with a CO faradaic efficiency of higher than 98% at a low overpotential of 0.37 V, and retains 98.5% of its initial selectivity after 50 h of electrolysis in 0.5 M KHCO3. In addition, the as-prepared catalyst exhibits enhanced selectivity of more than 95% from −0.5 to −0.8 V in 0.1 M KHCO3, has 98.6% CO faradaic efficiency at a low overpotential of 0.49 V, and retains 99% of its initial selectivity after 50 h of electrolysis, thus outperforming state-of-the-art Fe–N-based single-atom catalysts. Density functional theory calculations revealed that the atomic interfaces on the Fe clusters reduce the reaction barrier for the *COOH intermediate, thus contributing to the rapid transformation of CO2 molecules and resulting in excellent catalytic performance.


 Please wait while we load your content...
Please wait while we load your content...