Hydrogel-supported graphitic carbon nitride nanosheets loaded with Pt atoms as a novel self-water-storage photocatalyst for H2 evolution†
Abstract
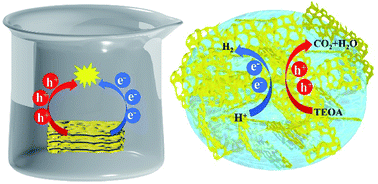
Graphitic carbon nitride (g-C3N4) exhibits an excellent photocatalytic performance as a powder, especially under visible light irradiation. However, it encounters great challenges for practical applications. For instance, to avoid aggregation and precipitation, a continuous stirring process is required for the bare g-C3N4 powder during the photocatalytic reaction. In addition, recycling of the powder photocatalyst is complicated and usually not environment friendly. To overcome these drawbacks, we present a hybrid materials. This material combines g-C3N4 nanosheets loaded with cocatalyst Pt atoms (CN) with a polymer hydrogel. In the ready-to-use photocatalyst, CN is well distributed in the hydrogel matrix. Water stored in the hydrogel can serve as a water reservoir for the photocatalytic water splitting. Due to the intermolecular interactions between CN and the hydrogel, a 3D network with a small-sized nanostructure is formed, which enhances the light absorption and the charge carrier separation. As a result, the H2 evolution rate is 7437 μmol h−1 g−1, which is 130% higher than that of the bare CN powder in water. Furthermore, the hydrogel-supported CN is able to function under ambient environment conditions without any significant reduction of the photocatalytic performance, as compared to the bare CN powder. In the hybrid material, the combination of hydrogel and CN provides a possibility for the photocatalyst to work without a water environment and accomplish an efficient H2 evolution.


 Please wait while we load your content...
Please wait while we load your content...