Using siloxane-based liquid electrolytes with high stability for fluoride shuttle batteries†
Abstract
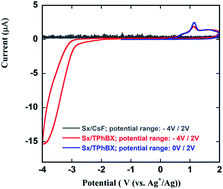
To benefit from the excellent safety as well as the thermal and electrochemical properties of siloxane-based liquid electrolytes, 2,2,4,4-tetramethyl-3,8,11,14,17-pentaoxa-2,4-disilaoctadecane (Sx) of the silsesquioxane family was used as an electrolyte for the first time in a fluoride shuttle battery (FSB) system. To overcome the solubility problem of a typical fluorine-based ionic salt (CsF) in Sx solvent, Sx was used in conjunction with an anion acceptor (triphenyl boroxine; TPhBX). The possible effects of Sx on the solubility of BiF3 in the electrolyte and on the electrochemical compatibility of the BiF3/C electrode for FSBs were fully investigated through cyclic voltammetry (CV) and charge–discharge tests at room temperature (RT) as well as at 55 °C. The related discharge and charge reactions were confirmed by X-ray diffraction analysis. The reaction potential of BiF3/Bi0 was investigated first in this study and was found to be −0.225 V vs. a standard hydrogen electrode (SHE) in Sx/TPhBX. A temperature increase from RT to 55 °C resulted in a lower IR drop and a more-efficient redox reaction at more positive potentials. Achieving a wider electrochemical stability window and reasonable ionic conductivity was speculated to have improved the FSB performance at 55 °C. In addition, Raman spectroscopy and atomic absorption spectroscopy (AAS) confirmed that BiF3 did not dissolve in the Sx/TPhBX system. The stable thermal and electrochemical properties of siloxane-based liquid electrolytes render them promising candidates as electrolytes that can be used at elevated temperatures.


 Please wait while we load your content...
Please wait while we load your content...