Tailoring desolvation kinetics enables stable zinc metal anodes†
Abstract
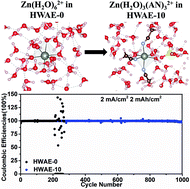
Low-cost and high-safety aqueous Zn ion batteries have been considered as promising alternatives to Li-ion batteries, provided that a stable Zn metal anode could be developed. Dendrite growth and low coulombic efficiency (CE) are the primary two issues afflicting the design of advanced Zn metal anodes. Inspired by complexing agents in the electroplating industry, acetonitrile (AN) is proposed as an electrolyte additive to guide the smooth growth of Zn. The enhanced intermolecular interactions between Zn2+ and mixed H2O/AN solvents lead to the supersaturating of adatoms on the current collector, as revealed by the complementary theoretical and experimental studies. Consequently, homogeneous nucleation and smooth growth of Zn are enabled for achieving exceptional stability up to 1000 cycles with an excellent CE of 99.64% on average. Application-wise, the incorporation of a complexing agent into the electrolyte is fully compatible with the cathode while maintaining the non-flammable nature for safe operation. The solvation chemistry regulation strategy provides a promising route to stabilize Zn metal anodes.


 Please wait while we load your content...
Please wait while we load your content...