Tartaric acid regulated the advanced synthesis of bismuth-based materials with tunable performance towards the electrocatalytic production of hydrogen peroxide†
Abstract
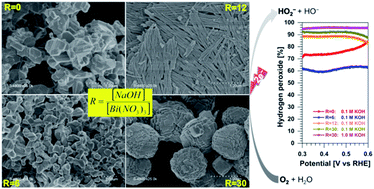
Tartaric acid has emerged as an ecofriendly organic compound for the biogenic preparation of micro- and nano-structured materials. For the synthesis mainly dictated by the coordination chemistry, questions remain open about the impact of the alkalinity on the nature of the resulting material as well as the properties. On the other hand, the design of advanced catalytic materials for the direct production of hydrogen peroxide from air is particularly needed. We report herein new synthesis, physico-chemical and electrocatalytic insights into bismuth-based materials. It was found that tight control of the alkalinity of the synthesis medium through the molar ratio R = [sodium hydroxide]/[bismuth(III) nitrate] leads to the production of a library of bismuth-based materials composed of metallic, (oxy)hydroxide, and oxide structures owing to the bismuth-tartrate complex intermediates. Electroanalytical studies show an oxygen-to-hydrogen peroxide selectivity in 0.1 M KOH of 77, 88 and 92% for R = 0, 12 and 30, respectively. In 1 M KOH, the selectivity is 90 and 96% for R = 12 and 30, respectively. The production rate is 69 ± 3 and 44 ± 3 mol kg−1 cm−2 over 2 h, corresponding to a faradaic efficiency of 92 ± 4 and 62 ± 5% for R = 30 and 12, respectively. These results place the present materials among the best known oxygen-to-hydrogen peroxide reduction catalysts without the need for preparing alloys or specific surface modification. This work contributes to engineering novel catalytic materials for advanced applications of on-site production of valuable chemicals.


 Please wait while we load your content...
Please wait while we load your content...