Autoxidation of polythiophene tethered to carbon cloth boosts its electrocatalytic activity towards durable water oxidation†
Abstract
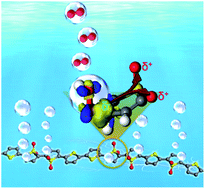
Cheap polymers are potential electrode materials for water splitting reactions due to their excellent mechanical properties and abundance. Much effort has thus been spent on exploring highly stable polymer electrodes especially for water oxidation, resulting in very little scope of polymers as potential candidates. Here, we use polythiophene with sulfur-based heterocycles to demonstrate the role of redox groups of unstable polymers in boosting both of its activity and stability as a polymer electrode for water oxidation. We found that the autoxidation of sulfur atoms in polythiophene tethered to the surface of carbon cloth greatly enhances the oxygen evolution reaction activity of the polymer carbon backbone by modifying its electronic structure, which isn't involved in the oxidation process and possible over oxidation, clarifying the pivotal role played by the chaperone redox groups.


 Please wait while we load your content...
Please wait while we load your content...