Layered electrides as fluoride intercalation anodes†
Abstract
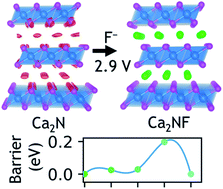
The fluoride ion is well suited to be the active species of rechargeable batteries, due to its small size, light weight, and high electronegativity. While existing F-ion batteries based on conversion chemistry suffer from rapid electrode degradation with cycling, those based on fluoride intercalation are currently less attractive then cation intercalation battery chemistries due to their low reversible energy densities. Here, using first-principles density-functional-theory calculations, we predict that layered electrides, such as Ca2N and Y2C – that have an electron occupying a lattice site – are promising hosts for fluoride intercalation, since their anionic electrons create large interstices. Our calculations indicate that anodes made from layered electrides can offer voltage up to −2.86 V vs. La2CoO4 cathode, capacity >250 mA h g−1, and fast diffusion kinetics with migration barriers as low as 0.15 eV. These metrics compare favorably to popular Li-ion intercalation cathodes such as LiCoO2. Electrides open up a new space for designing fluorine intercalation batteries with good performance and cyclability.


 Please wait while we load your content...
Please wait while we load your content...